Choosing a format and dose
1 of 3
Have you used CBD before?
Select one of the following options
Your recommendation dosage is:
Recommended dosage:
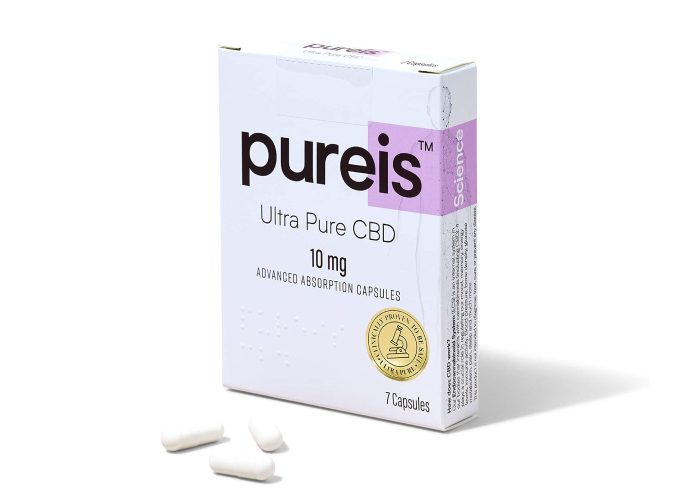
Here’s a product you can use to get started:
Here are products you can use to get started:
Discover the science of CBD
Why Pureis® CBD?
Pureis® was founded by Chanelle McCoy and Caroline Coen, both with extensive pharmaceutical training and experience.
When they became aware of the many misleading and poor-quality CBD products on the market, they resolved to develop a safe, certified product for the consumer. The rest is Pureis® history.

.png)


.png)

Why [Pureis®] Lab Made CBD?
CBD can be made in one of two ways:
1. Processing cannabis plants - isolating CBD and attempting to remove all impurities;
2. Synthesising pure CBD in a laboratory.
It is nearly impossible to extract pure CBD from the cannabis plant. There will always be traces of other cannabinoids, including THC, the addictive part of cannabis.
Pureis® is made by scientists, using citrus fruits to produce pure CBD by mimicking specific sections of DNA from the cannabis plant. Because there are no impurities to begin with, there are no impurities in the end product. All you get is completely safe, Ultra Pure CBD.

Shop now
@pureiscbd
Error: No feed with the ID 1 found.
Please go to the Instagram Feed settings page to create a feed.